Chemical Sciences
Applications for 2025-2026 open on 1 July 2025
Characterisation of beverage antioxidants using cyclic voltammetry
Project code: SCI001
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
The antioxidants present in beverages can be quantified and information provided about their reducing strength using the electrochemical technique of cyclic voltammetry. This technique has been developed at the University of Auckland to profile wines, fruit juices, teas and coffees, and milk.
The role
In this project, the voltammetry procedure will be applied to the antioxidants present in a series of alcoholic beverages, including beer and fortified drinks. An examination of the most appropriate solvent for the measurement of the phenolic and other antioxidants present will be made, along with the electrode conditions needed to make a reliable quantification. Comparisons will be made with standard Food Science antioxidant assays, and a wide range of beverages of different strengths will be surveyed.
Behaviour of platinum electrodes at open circuit in grape juice and wine, compared to O2 content
Project code: SCI002
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
The so-called redox potential of wine at a bright platinum electrode (with no current flowing) has a long history in enology. However, our own experiments have indicated that the measure provides only a broad indication of dissolved oxygen content, but little further information on wine redox status.
The role
The experimental tests will include monitoring the redox potential and the dissolved oxygen content simultaneously during fermentation. Selective additions of ethanol to wines will be made and the redox potential response recorded. Prolonged exposure of wine to Pt at open circuit will be undertaken to check for acetaldehyde formation.
Compare commercial disposable PEDOT electrodes with home-made PEDOT for ascorbic acid oxidation
Project code: SCI003
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
Past projects at the University of Auckland have shown that PEDOT is a reliable redox mediator for the analysis of small molecule antioxidants.
At our own PEDOT electrodes, signals for ascorbic acid are well separated from those due to uric acid or catechol-containing polyphenols, which appear at higher electrode potentials. However, the commercial PEDOT electrodes do not seem to perform so well, and a cross-comparison is needed, both with ascorbic acid and with other target antioxidants.
Dynamic Microfluidics using High-Speed Photography
Project code: SCI004
Supervisor(s):
Discipline(s): School of Chemical Sciences

Project
The Dynamic Microfluidics Laboratory (https://fluidics.physics.auckland.ac.nz/) uses high-speed photography to study the motion of fluids at small length scales.
The role
Various projects are available to study phenomena including (for example):
- Impacts of drops on to surfaces, especially fluids of industrial interest, which are often non-Newtonian (e.g. milk, ferrofluids).
- The fate of droplets floating in air, relevant (for example) to the spread of viruses.
- The detailed dynamics of capillary uptake.
Ideal student
The student should be an aspiring chemist with an interest in analytic, physical/materials, food, or forensics topics.
Skills gained
They will gain skills and experience relating to materials characterization, high-speed photography and/or image analysis.
Janus Spheres
Project code: SCI005
Supervisor(s):
Discipline(s): School of Chemical Sciences
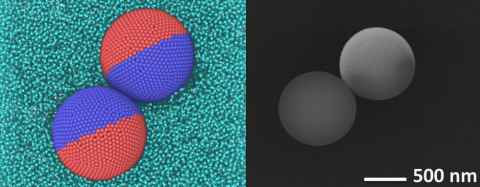
Project
This project will study Janus spheres, which are microspheres which have two (or more) different surface coatings applied to them. We are interested in how the asymmetry of these spheres affects the formation of self-assembling particle clusters. Janus particle clusters could be used to develop reconfigurable components for sustainable technologies, and they also serve as a good model for biological self-assembly.
The role
To study clustering, particles in solution are observed as they come together on a microfluidic chip.
Ideal student
The student should be an aspiring physical/materials chemist, who will gain skills and experience relating to materials characterization, microfluidics, fabrication, and/or image analysis methods.
Lab website
https://fluidics.physics.auckland.ac.nz/
How squishy is a soft particle?
Project code: SCI006
Supervisor(s):
Discipline(s): School of Chemical Sciences
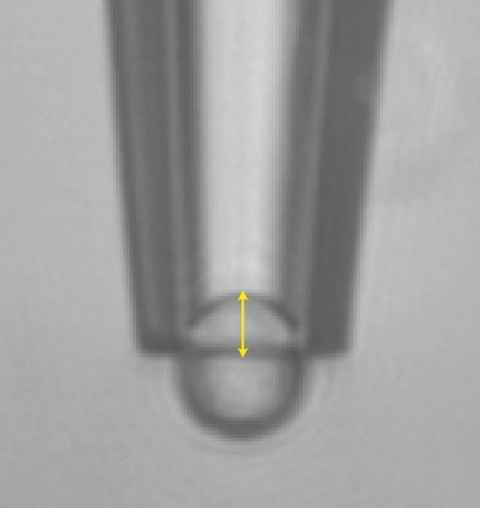
Project
We have developed a method (known as ‘aspiration’) which can measure the mechanical properties of soft microparticles using the humble pipette. The important role of such mechanical properties in (bio-)medical research is emerging.
The role
Projects are available to apply aspiration to interesting particles. For example, we are keen to explore the mechanics of liposomes, a family of synthetic micro- and nanoparticles analogous to cells, and with potential drug delivery applications.
Ideal student
The student should be an aspiring physical/materials scientist, who will gain skills and experience relating to materials fabrication and characterization.
Lab website
https://fluidics.physics.auckland.ac.nz/
Flexible conductive hydrogel-based electronic skin (E-skin) for wound healing applications
Project code: SCI007
Supervisor(s):
Discipline(s): School of Chemical Sciences
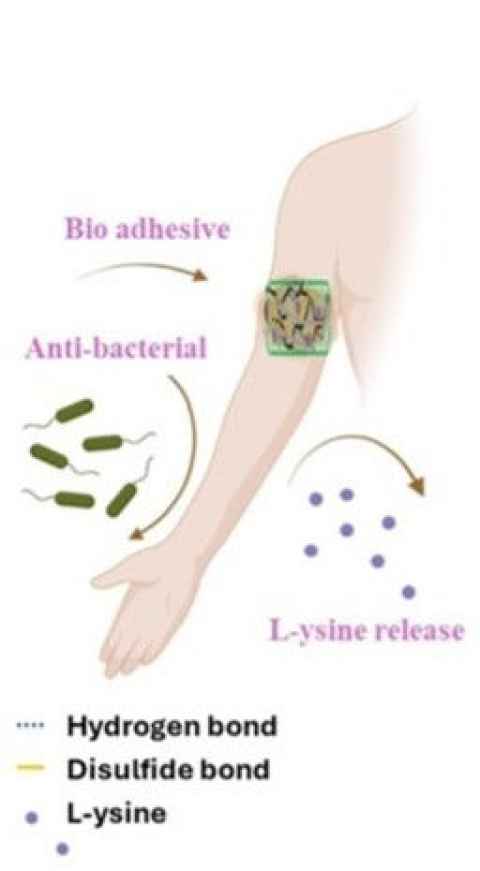
Project
Flexible conductive hydrogels for electronic skin (E-skin) are gaining attention for their potential in combined therapeutic effects on both drug delivery and electrical stimulation.
The role
This project involves the development of a uniform Lysine/THA/PPy-BC hydrogel. In this system, lysine electrostatically interacts with self-crosslinking thiolate hyaluronic acid (THA) to form a stable network, which is subsequently incorporated into a polypyrrole-bacterial cellulose (PPy-BC) hydrogel.
The student will be involved in the optimization of the system to achieve the best composition for mechanical properties and drug release profile.
Biodegradable Conducting Polymers for Transient Electronics
Project code: SCI008
Supervisor(s):
Discipline(s): School of Chemical Sciences
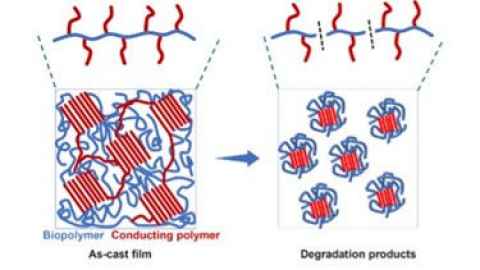
Project
This summer project will focus on developing transient electronics using the biopolymer chitosan and a conducting polymer through a simple fabrication method.
Skills gained
Student will gain experience in polymer processing, film casting, electrical measurements and evaluating material degradation. Prior knowledge in chemistry or materials science is desired.
Reference
ACS Applied Materials & Interfaces, 16, 2024, 23872
Aptamer-based Sensor for Detection of Saxitoxin
Project code: SCI009
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
Saxitoxin is a highly potent neurotoxin primarily known as a paralytic shellfish toxin. This study will investigate how varying concentrations of the saxitoxin-specific bioprobe, M30f aptamer, affects the sensor’s response in electrochemical detection of Saxitoxin.
Ideal student
Understanding of basic electrochemistry would be of advantage as well as an interest in materials science.
Paper-based laser induced graphene for bioelectronics
Project code: SCI010
Supervisor(s):
Discipline(s): School of Chemical Sciences
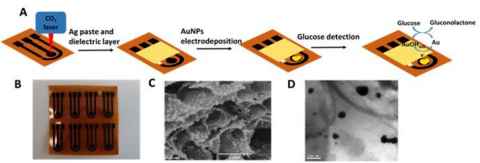
Project
This project involves the preparation of paper based laser induced graphene (LIG), which would be fabricated into sensors for the detection of glucose and dopamine.
The role
Students will design electrode patterns, optimize the laser cutting parameters and print various electrodes via laser cutting machine. Electrochemical tests will be conducted to evaluate the performance of electrodes.
Ideal student
No prior experience required- basic lab skills and strong interest in materials science preferred.
Figure shows fabrication of a disposable glucose sensor fabricated by laser scribing (Electrochimica Acta, 378, 2021, 138132).
Stimuli-responsive hydrogel beads for selective dye adsorption
Project code: SCI011
Supervisor(s):
Discipline(s): School of Chemical Sciences
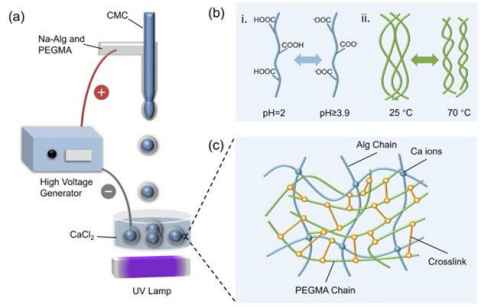
Project
This project involves preparing alginate–PVA hydrogel beads and testing their adsorption of cationic and anionic dyes (e.g., methylene blue and amaranth dyes) across pH 2–7.
The role
Students will conduct UV-Vis measurements and apply kinetic models to evaluate adsorption efficiency.
Ideal student
No prior experience required; basic lab skills and interest in materials science preferred.
Figure shows hydrogel capsules’ fabrication by electrospraying and their response to pH and temperature (Journal of Colloid and Interface Science, 677, 2025, 942).
Exploring the Stability of Protecting Groups in Surface Organometallic Chemistry
Project code: SCI012
Supervisor(s):
Discipline(s): School of Chemical Sciences
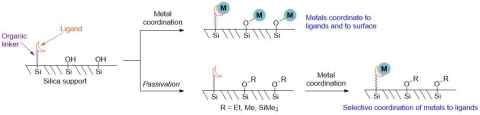
Project
Surface Organometallic Chemistry attaches transition metal coordination compounds onto solids such as silica. Our aim is to make catalysts for more sustainable industrial chemical production.
We synthesise silica supports with ligands attached, which coordinate to metals. However, silica is also covered in Si-OH groups, which compete with ligands to react with metals.
The role
We need to add protecting groups to prevent unwanted side reactions with metals. In this project, you will investigate the stability of silica protecting groups under a variety of conditions.
Ideal student
This project is most suited to students with some prior laboratory experience and an interest in inorganic chemistry.
Synthesising Silica Functionalised with Amines
Project code: SCI013
Supervisor(s):
Discipline(s): School of Chemical Sciences
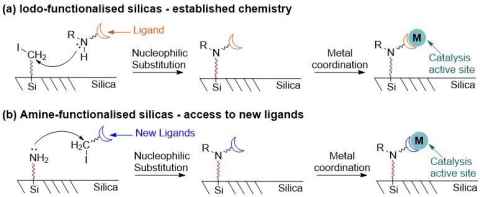
Project
In the Falconer group, we affix transition metal coordination compounds to solid supports to make sustainable catalysts suitable for industrial scale.
In the Falconer group, we synthesise silicas with functional groups, transform them to ligands and coordinate to metals. Whilst it is well established how to make silica with iodo-groups for reacting with nucleophiles, making silicas with amines attached (for reaction with electrophiles) is more difficult.
The role
In this project, you will make amine-functionalised silicas, which will allow us to access to a wider range of ligand attached to solid supports.
Ideal student
This project is most suited to students with some prior laboratory experience and an interest in inorganic chemistry.
Safe-by-design alternatives to forever chemicals
Project code: SCI014
Supervisor(s):
Dr Mitch Nascimento
Discipline(s): School of Chemical Sciences
Project
Upwards of 10 000 per- and poly-fluoroalkyl substances (PFAS) are widely produced and applied due to their non-stick, as well as chemical-, water-, heat- and stain-resistant properties. These amazing features also result in their environmental mobility, persistence, and bioaccumulation, leading to adverse human health effects and government bans. Therefore, there is an urgent need to replace essential use PFAS.
The role
In this research project you will synthesize and characterise alternatives to PFAS, as well as test their properties.
Safe-by-design alternatives to forever chemicals
Project code: SCI015
Supervisor(s):
Dr Mitch Nascimento
Discipline(s): School of Chemical Sciences
Project
Silicone-based materials are ubiquitous due to their unique properties, such as biocompatibility, flexibility, tunability, and thermal resistance. These properties result in their use across all sectors, from healthcare to consumer goods to building and construction.
One of the challenges with silicone is recyclability as, unlike some other polymers, it is not possible to heat and remould them.
The role
In this project you will be involved in synthesizing, characterizing, and degrading modified silicone which is designed for easy recycling.
Using the composition of deep eutectic solvents to control acidity
Project code: SCI016
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
Deep eutectic solvents (DESs) are mixtures that have melting points below each of the constituents of the mixture, forming room temperature liquids from solid components. This allows solvents to be made from non-toxic, bio-based compounds that are not inherently liquid.
Recently, we have discovered that the composition of these liquids can significantly affect their ability to extract transition metals from waste streams, which we believe is linked to changes in the ionisation of carboxylic acid components of the mixture. This implies the ability to modify the acidity of the DES through its composition, an effect that could help tailor DESs for a range of potential applications.
The role
The aim of this project will be to understand the relationship between the composition of selected DESs and their acidity, by preparing and measuring the acidity of a range of structurally diverse DESs. This will open up the possibility of tuning DES acidity for selected applications, including their role as extractants for metal-containing waste and as benign acid catalysts for chemical transformations.
Protic ionic liquids for biomass transformations
Project code: SCI017
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
Ionic liquids, low melting salts, display unique capabilities with regard to the transformation of biomass components such as biopolymers and sugars into potential platform chemicals. Unfortunately, many of the ionic liquids used for these transformations are inherently expensive and lead to significant embedded environmental harm owing to the number of synthetic steps required in their preparation.
The role
This project will investigate the use of low cost protic ionic liquids easily prepared from abundant reagents as solvents and promoters for the preparation of bio-based platform chemicals from precursors such as chitin (or its monomer N-acetylglucosamine) and cellulose (or its monomer glucose).
Developing greener switchable solvent systems
Project code: SCI018
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
The development of non-volatile solvents has been a long-standing aim of green chemistry given the hazards and environmental harm caused by volatile organic solvents. While many non-volatile liquids have been developed as solvents, recovering compounds dissolved in these solvents has been a challenge and limited their uptake.
Switchable solvents, liquids that abruptly change polarity when exposed to a stimulus such as CO2, temperature, pH or light, have been proposed as a solution. These systems can undergo significant changes in polarity or miscibility with other solvents (such as water) which can allow the recovery of solutes, without needing to rely on the distillation of a volatile solvent.
The role
Unfortunately most switchable solvents to date are based on highly toxic amines which needs to be addressed to enable them to be a truly green solution. This project will investigate approaches to develop greener switchable solvents by exploring the potential of safer amine components and investigating novel amine-free switchable systems.
Bioorganometallic Anticancer Chemotherapeutics: Preparation of Metal Complexes with Bioactive Ligands
Project code: SCI019
Supervisor(s):
Discipline(s): School of Chemical Sciences
Project
Metal complexes are used in the treatment of about 70% of cancer patients but they cause side effects and are not suitable to treat every type of tumour, also tumours develop resistance. The coordination of bioactive ligand systems to metal centres results in multimodal anticancer agents, i.e., anticancer drugs that have more than one of mode action.
The role
This design strategy is a promising route to overcome the major limitations of current cancer chemotherapeutics. We will develop in this project new complexes using organometallic moieties and bioactive ligand systems and study their anticancer activity.
Design of Multimodal Organometallic Anticancer Agents
Project code: SCI020
Supervisor(s):
Discipline(s): School of Chemical Sciences
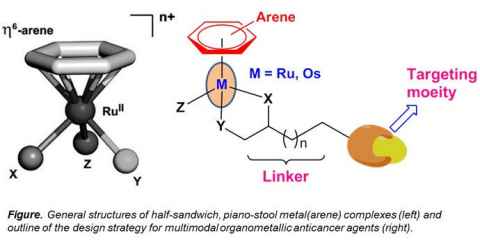
Project
In the past decade the design of targeted anticancer agents was among the most prolific research areas. However, more recently it has become apparent that the combination of more than one pharmacophore in a single molecule can result in anticancer agents with advantageous properties.
The role
In this project, we will work on the preparation of a new compound class to be tested on its tumour-inhibiting properties. We will combine an anticancer active ligand system with a metal centre and characterize the compounds using a wide variety of methods.
Bio-inspired Iron Complexes for Water Purification
Project code: SCI021
Supervisor(s):
Discipline(s): School of Chemical Sciences
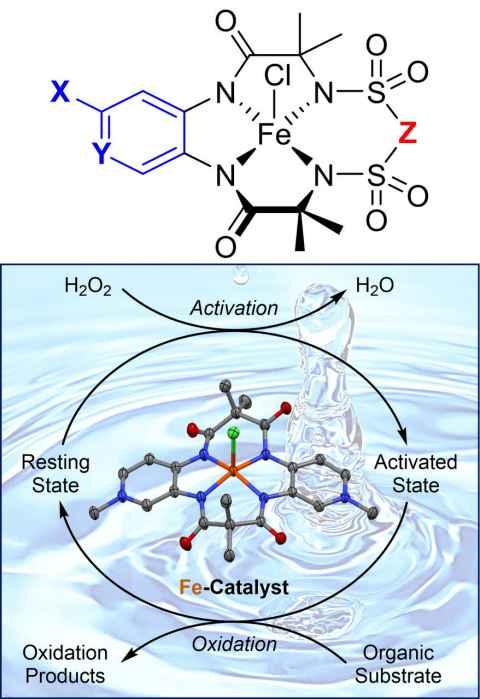
Project
Treated wastewater released to the environment contains harmful micropollutants. This is recognised as a very serious problem, especially in view of the massive scale involved (globally ca. 188 billion cubic meters/year). Proposed final-step purification approaches all have problems, e.g., high cost, low selectivity and the generated waste.
The role
We will synthesize non-toxic, iron-based, bio-inspired complexes that will catalyse micropollutant oxidation by hydrogen peroxide at nanomolar concentrations to give water that is environmentally safe.
Lithium Complexes for the Treatment of Bipolar Disorder
Project code: SCI022
Supervisor(s):
Discipline(s): School of Chemical Sciences
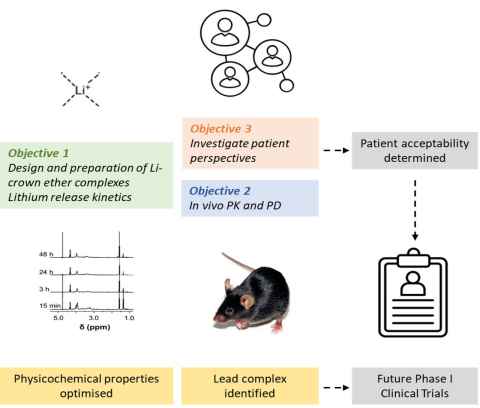
Project
Lithium is the gold-standard treatment for bipolar disorder and the only medication to reduce suicide risk. However, its effective dose is close to the toxic dose and frequent blood monitoring is required to prevent poisoning. Around half the individuals taking lithium stop their treatment due to adverse effects including kidney damage, weight gain and cognitive impairment.
The role
We will prepare lithium compounds to improve delivery with a quicker response and fewer side effects than current formulations. We will investigate the stability and release kinetics.
Supramolecular structures and their use for targeted delivery of anticancer agents
Project code: SCI023
Supervisor(s):
Discipline(s): School of Chemical Sciences
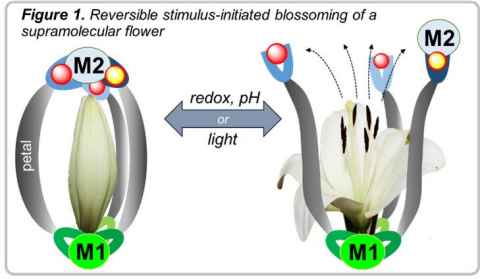
Project
Stimulus responsive supramolecular structures may provide a means to deliver anticancer agents selectively to the tumour and the stimulus can be used to release an anticancer drug from the structure.
The role
In this project, we will prepare ditopic ligands that will be coordinated to metal centres to form supramolecular structures. The supramolecules will be characterised and their ability to host and release anticancer drugs will be assessed.
Investigating the Environmental DNA Isolation Using Conductive Polymers
Project code: SCI024
Supervisor(s):
Dr Alireza Akbarinejad
Discipline(s): School of Chemical Sciences
Project
This project investigates how different dopants in conductive polymers affect the capture and release of environmental DNA (eDNA).
The role
Building on preliminary findings, the student will synthesise polymers with various dopants and assess their performance. The work includes hands-on experience with material synthesis, DNA handling, and characterisation techniques such as SEM, EDS, Raman, FTIR, and water contact angle measurements.
Ideal student
The project is ideal for a summer student interested in materials science and environmental applications of polymer-based systems.
Investigating the Role of Electrolytes in Electrochemical eDNA Capture and Release
Project code: SCI025
Supervisor(s):
Dr Alireza Akbarinejad
Discipline(s): School of Chemical Sciences
Project
This project explores how different electrolytes affect the efficiency of environmental DNA (eDNA) capture and release from conductive polymers using electrical stimulation.
The role
The student will test various electrolytes and evaluate their impact on DNA binding and release. The project also aims to investigate the mechanisms behind these effects.
Skills gained
Students will gain hands-on experience in electrochemical techniques, DNA analysis, and basic materials characterisation.
Ideal student
It is ideal for those interested in materials science, surface chemistry, and environmental applications.
Enhancing eDNA Capture Efficiency Using Conductive Polymer Hydrogels
Project code: SCI026
Supervisor(s):
Dr Alireza Akbarinejad
Discipline(s): School of Chemical Sciences
Project
This project aims to improve environmental DNA (eDNA) capture efficiency by applying a conductive polymer-loaded hydrogel layer onto carbon cloth substrates. The hydrogel is expected to increase the available surface area for DNA binding, addressing limitations observed with the current system.
The role
The student will focus on hydrogel fabrication, polymer integration, and evaluating electrochemical DNA capture performance.
Skills gained
The project provides hands-on experience in materials development, surface engineering, and basic material characterisation techniques such as SEM, EDS, and mechanical testing.
Designing sweet molecules with machine learning and physics-based pipelines
Project code: SCI027
Supervisor(s):
Dr Katerina Taškova
Discipline(s): School of Chemical Sciences
Project
This project will revolve around designing sweet-tasting proteins using machine learning (ML) at the intersection of computational biochemistry and AI.
The role
The successful candidate will apply structural bioinformatics, molecular modelling, and ML algorithms to predict and optimize protein–taste receptor interactions: leveraging the recent publication of the first 3D structure of the human sweet taste receptor.
Skills gained
Students will gain experience with protein sequence analysis, 3D structure prediction and pipelines for protein design.
Ideal student
Ideal for students with backgrounds in biology, chemistry and/or computer science, eager to apply interdisciplinary skills to address real-world challenges in sustainable healthy solutions.
This project is in collaboration with US scientists specialised in AI-based protein design, opening up opportunities to network.
Understanding how machines learn to sample protein shapes and function
Project code: SCI028
Supervisor(s):
Discipline(s):
School of Chemical Sciences
School of Computer Science
Project
This project will investigate how machine learning (ML) models learn to predict and simulate protein structures and infer their function from structural data.
The role
This project introduces students to key concepts in ML/deep learning, 3D data representation, and protein structure-function relationships. You will work with tools for statistical data analysis, and ML learning frameworks for clustering and classification of protein molecules, alongside protein sequence and structure datasets, to explore how algorithms:
i) "See" and classify molecular features for drug design
ii) Assert molecular functions and dysfunctions in disease
Ideal student
This project is ideal for students with interests in AI, bioinformatics, or structural biology who want to bridge the gap between computational models and biological insights.
This project is in collaboration with academics in Denmark specialised in smFRET spectroscopy, opening up opportunities to network.
How does a single mutation of a drug transporter affects Pacific and Māori populations? A molecular dynamics simulations study
Project code: SCI029
Supervisor(s):
Discipline(s):
School of Chemical Sciences
Project
The recent discovery of a single mutation over-represented in Pacific and Māori populations has been linked to an impaired absorption of drugs prescribed to fight diabetes and obesity.
The role
The successful research candidate will run and analyse molecular dynamics simulations of the proteins carrying the mutation, to investigate the root causes of impaired drug absorption.
Skills gained
The candidate will be fully trained in running and analysing molecular simulations with python-based frameworks.
Ideal student
This project does not require previous knowledge of simulations and is suited for a candidate with interests in computational biophysics, and with a background of medicinal/biological chemistry, biology, physics or computer science.
Plasma Jet Printing of Metal Oxide Composites
Project code: SCI030
Supervisor(s):
Discipline(s):
School of Chemical Sciences

Project
Non-equilibrium (“cold”) atmospheric pressure plasmas are an emerging technology that provides an alternative deposition route to those commonly employed for thin film fabrication. Cold atmospheric pressure plasmas eliminate the need for vacuum systems (often used in CVD setups), organic binders (screen printing) and/or high thermal temperatures.
The role
In this project we aim to develop a simple one-step deposition of mixed metal oxide composite films using a cold atmospheric pressure plasma jet.
Allosteric modulators of cannabinoid and opioid GPCRs
Project code: SCI031
Supervisor(s):
In collaboration with Prof Michelle Glass (Otago)
Discipline(s):
School of Chemical Sciences

Project
G-protein coupled receptors (GPCRs) are integral membrane bound proteins (A) that detect external stimuli and activate cellular signalling pathways. Important examples include sight (rhodopsin GPCR) and analgesia (mu opioid GPCR). A large percentage of current drugs target GPCRs, making them central to the discovery of next generation therapeutics.
The role
This project focuses on the design, synthesis and evaluation of new allosteric modulators for the cannabinoid and opioid GPCRs (CB1 and MOR). These ligands bind away from the active site, allowing for the preservation of normal timing and distribution of cell signalling, leading to novel drugs with higher selectivity, reduced side effects and unique cell signalling profiles (B).
Ideal student
You will be keen to develop skills in organic synthesis and drug design. Students with skills or interest in pharmacology and molecular modelling are also encouraged to get in touch, to discuss project branches emphasizing those areas.
New reaction discovery, in cycloaddition-promoted rearrangement chemistry
Project code: SCI032
Supervisor(s):
Discipline(s):
School of Chemical Sciences
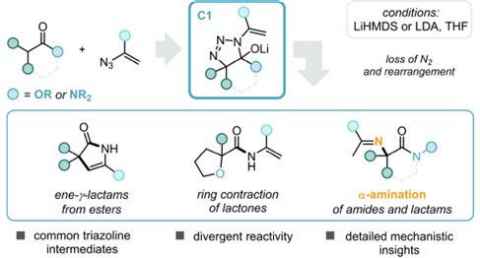
Project
We have shown in our group that enolates and azides undergo facile (3+2) cycloaddition reactions, followed by rearrangement to give a wide variety of useful molecular scaffolds (see Figure) from simple starting materials.
The role
This project focuses on the evaluation of new substrate types, to further probe the possibilities of the reaction manifold for generation of different product architectures. Many of the products available through this chemistry are of high potential value in drug discovery and natural product synthesis, and would be difficult to access in other ways. Some examples of applications in natural product or drug synthesis will also be pursued.
Ideal student
You will be keen to develop skills and theory in organic synthesis and reaction design. Students with skills or interest in molecular modelling are also encouraged to get in touch, to discuss project branches emphasizing those areas.
Virus Activated Cancer Prodrugs
Project code: SCI033
Supervisor(s):
Discipline(s):
School of Chemical Sciences
Ngā Motu Whakahī
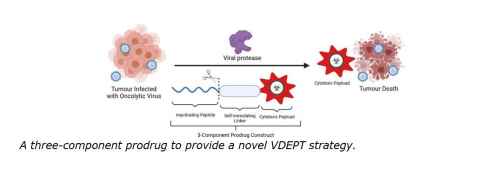
Project
Oncolytic viruses are an emerging class of therapeutics for cancer treatment. These viruses selectively infect and lyse cancerous cells. However, these therapies still suffer from certain limitations, perhaps the greatest of clearance of the virus prior to complete tumour destruction due to the patient’s immune system. To elicit maximal efficacy, these viruses can be used in combination with chemotherapeutics or radiotherapy.
The presence of viral infection provides new opportunities to develop selectively targeted chemotherapeutics.
The role
This project seeks to develop a novel Virus-Directed Enzyme Prodrug Therapy (VDEPT). Cytotoxic payloads will be developed and conjugated to an inactivating peptide sequence that is selectively cleaved by the protease of a promising oncolytic virus to release the active cytotoxin selectively in the tumour microenvironment.
Thus, the project seeks to develop a novel prodrug and combination therapy. A key aspect of the research will be optimising the self-immolating cleavable linker system for a favourable rate of payload release.
See image
Skills gained
The project is an active collaboration with the University of Otago. The successful candidate will develop skills in modern organic chemical synthesis, Solid Phase Peptide Synthesis (SPPS), reverse phase-HPLC and may also have the opportunity to conduct biological assays/enzyme assays.
Stapling Antimicrobial Peptides to Treat Internalised Group A Streptococcus Infection
Project code: SCI034
Supervisor(s):
Discipline(s):
School of Chemical Sciences
Ngā Motu Whakahī
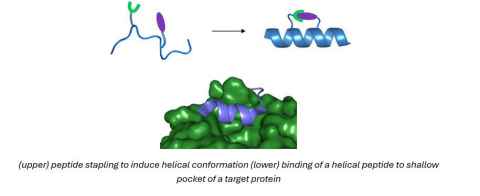
Project
Stapled peptides are an emerging class of therapeutics that bridge the gap between small molecule drugs and biologicals (e.g. monoclonal antibodies – Herceptin), allowing one to target protein-protein interactions (PPIs) once considered “undruggable”. Using modern organic synthesis techniques, linear peptides can be “stapled” to improve their α-helical secondary structure and biological activity properties. Stapled peptides benefit from enhanced receptor affinity and selectivity, improved membrane permeability (accessing intracellular targets) and increased half-lives in body.
This project will develop new stapling methods and a SAR study of stapled-antimicrobial peptides (AMPs) to treat an NZ relevant bacterial pathogen, Group A Streptococcus. This pathogen internalises itself inside host epithelial cells and a defence mechanism, allowing it to hide from the immune system and antibiotics, most of which are not permeable to human cells. This results in treatment failure, recurring infection and severe complications such as rheumatic heart disease.
The role
Using novel peptide stapling approaches, you will develop cell permeable stapled antimicrobial peptides which will be tested for activity towards bacteria internalised by epithelial cells.
See image
Skills gained
Successful candidates will use organic synthesis techniques and modern methods of solid phase peptide
SAR Study to Combat Antimicrobial Resistance
Project code: SCI035
Supervisor(s):
Distinguished Professor Dame Margaret Brimble
Discipline(s):
School of Chemical Sciences
Ngā Motu Whakahī
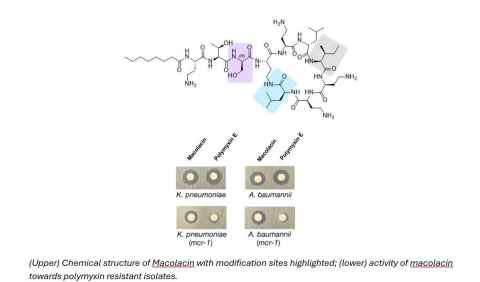
Project
Antibiotic resistance is recognised by the WHO as one of the greatest threats to humanity, and infectious diseases rank as the second most common cause of death worldwide.
Polymyxin antibiotics are the current last-line of defence, but are severely nephrotoxic. Most worryingly, since 2015, a mobile resistance gene (mcr-1) has been spreading globally and making our last hope in the clinic ineffective. In 2022, macolacin was discovered. Macolacin is a new polymyxin scaffold that retains potent activity towards mcr-1 mediated polymyxin resistant Gram-negative bacteria.
See image
The role
This project seeks to conduct a structure-activity-relationship (SAR) study of macolacin and prepare new analogues with diminished toxicity that could replace polymyxins as last-line of defence antibiotics in the clinic. Successful candidates will use organic synthesis techniques and modern methods of solid phase peptide synthesis. Candidates will also have the opportunity to undertake and learn biological assays if they desire.
Studying Protein Post-Translation Modification
Project code: SCI036
Supervisor(s):
Discipline(s):
School of Chemical Sciences
Ngā Motu Whakahī
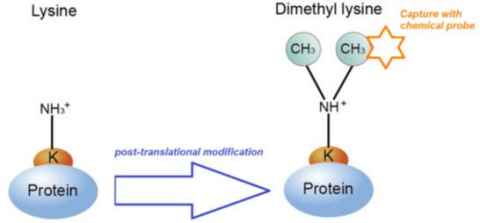
Project
Post-translational modification (PTM) is a crucial process that gives proteins their final conformation and chemical properties, thereby determining their biochemical functions. The recent recognition of the significant role that PTMs play in both health and disease has sparked increased interest in this research field.
Of these, methylation is common PTM that occurs on histones, the proteins which provide structural support to chromosomes. Methylation of histones is known to regulate transcription of genes and accordingly, has a role in almost all cellular processes, e.g. cell cycle, stress response etc.
The role
As a result, histone methylation plays a role in lifespan, ageing and disease. We are particularly interested to study dimethylation of lysine residues and its occurrence in different disease. This research project will using recent advances in chemical methods from our laboratory to develop a chemical probe to selectively label and study dimethyl-lysine PTMs in disease. The project will combine synthetic chemistry and analytical chemistry techniques (e.g. HPLC and mass spectrometry) to develop this new platform, with the ultimate goal of answering fundamental questions in biological science an health research.
Development of Earth-Abundant Photosensitisers
Project code: SCI037
Supervisor(s):
Discipline(s):
School of Chemical Sciences
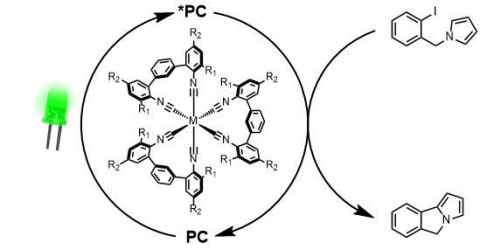
Project
We are seeking to develop new photosensitisers based on earth-abundant first-row transition metal complexes, as sustainable and cost-effective alternatives to precious metal photosensitisers.
The role
In these projects, we will design and prepare novel photoactive complexes, spectroscopically investigate their photophysical properties, and test their performance in photoredox and energy-transfer catalysis applications.
These projects can be tailored to student interests in coordination chemistry, photochemistry, spectroscopy and photochemical natural products synthesis.
Photoresponsive Molecules for Targeted Drug Delivery
Project code: SCI038
Supervisor(s):
Discipline(s):
School of Chemical Sciences
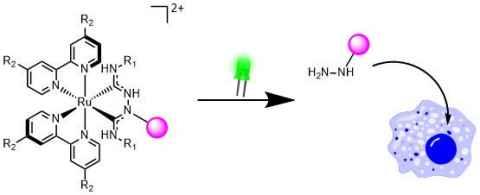
Project
We are seeking to develop novel Ru-based photoresponsive molecules that selectively deliver anti-cancer drugs to tumours, released with high spatiotemporal control through non-harmful visible light irradiation – thus circumventing the unpleasant side-effects of traditional chemotherapy.
Offered projects include:
- Developing and studying new photocage scaffolds with novel photorelease mechanisms
- Developing new methods of connecting drug molecules / stimulants to photocages
- Investigating photorelease mechanisms through temperature-dependent spectroscopy
Supramolecular Approaches Towards Artificial Photosynthesis
Project code: SCI039
Supervisor(s):
Discipline(s):
School of Chemical Sciences
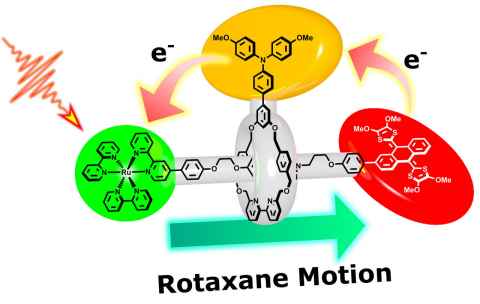
Project
We are investigating new paradigms to transport charge at the molecular scale using the dynamic nature of supramolecular architectures known as molecular machines. In doing so, we seek to overcome current limitations in biomimetic artificial photosynthesis and successfully mimic key steps of natural photosynthesis.
Offered projects include:
- Developing and studying rotaxanes that transport charge via molecular machine-type behaviour
- Developing synthetic approaches to higher order rotaxanes for long-range charge separation
Modernising Classic Experiments with Chemometric Analyses
Project code: SCI040
Supervisor(s):
Discipline(s):
School of Chemical Sciences
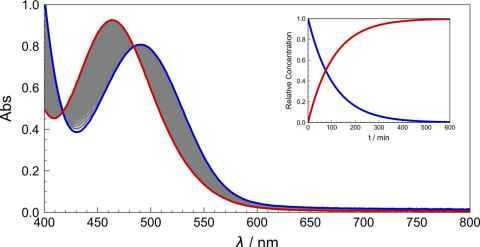
Project
In this project, we are seeking to modernise classic undergraduate laboratory experiments with the state-of-the-art spectroscopic facilities in our undergraduate labs – and through the utilisation of now widespread digital tools to perform powerful chemometric analyses that will enhance student understanding of underlying theoretical concepts.
The role
These experiments are generally focussed around the use of UV-Vis spectroscopy to investigate reaction rate constants, thermodynamic binding constants and pKa values. Chemometric analyses will be implemented across several platforms including Python, R and Matlab.
Caging Functional Molecules to Understand Communication in Brain Cancer Cells
Project code: SCI041
Supervisor(s):
Prof. Charles Unsworth
Discipline(s):
School of Chemical Sciences
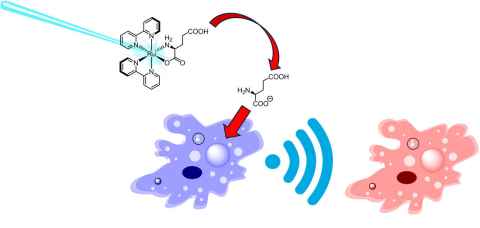
Project
We are seeking to study how brain cancer cells communicate in 3D. To achieve this, we will introduce ‘caged’ biostimulants (e.g. glutamate) that can be released under light irradiation with high spatiotemporal control to stimulate a single cell, and track how the cells communicate using 3D fluorescence microscopy techniques.
The role
This project will involve the synthesis and characterisation of coordination compounds with ligated glutamate and potentially other biostimulants, and study their light-induced release from photoactive Ru(II) complexes for cell communication studies.
Squishing cells and vesicles
Project code: SCI056
Supervisor(s):
Discipline(s):
Ngā Motu Whakahī
School of Chemistry
School of Physics
Project
This scholarship will be hosted by Ngā Motu Whakahī and is specifically targeted toward students who whakapapa Māori or are of Pacific heritage.
A funded project is available to study soft microparticles (e.g. cells) being squeezed within a constriction (e.g. a pipette tip). This work is part of a project to develop new methods for easily analysing the mechanical properties of soft particles.
The role
The project may be experimental or computational, depending on the student’s interests and skills.
For example, an experimental project could work on collecting and analysing data for a particular set of soft particles. A theory / computational project could work on models that are used to find mechanical properties based on experimental data.
Lab website: https://fluidics.physics.auckland.ac.nz/
Monitoring Prostate Health in Aotearoa using Wastewater-based Epidemiology
Project code: SCI062
Supervisor(s):
A/Prof Lisa I. Pilkington (Associate Professor)
Miriama Wilson (PhD student) - Ngāti Kahungunu ki Wairoa, Ngāti Tūwharetoa, Ngāti Raukawa
Discipline(s):
Ngā Motu Whakahī
Biological Sciences
Project
This is a Ngā Motu Whakahī scholarship specifically targeted toward students who whakapapa Māori or are of Pacific heritage.
Wastewater-Based Epidemiology (WBE) involves the chemical analysis of wastewater for biomarkers to gain information on the habits and health of a population.
This project will involve identifying and quantifying the prostate health biomarker, Prostate Specific Antigen (PSA) in wastewater from around Aotearoa. We hope to compare PSA levels for each of the 21 catchments being sampled, to direct health resources and support.
Skills preferred (but can be taught)
Analytical chemistry skills, including Liquid Chromatography – Mass Spectrometry (LC-MS) and wet laboratory skills.